THE KINETIC STUDY OF CO2 REFORMING WITH CH4 USING La2O3 PROMOTED COBALT SUPPORTED ALUMINA CATALYST
Main Article Content
Abstract
The CO2 reforming reaction has recently emerged as a promising method for producing syngas from CO2-rich natural gas. Whereas the Co-based materials with the addition of promoter appeared to be potential catalysts and gained much attention since they not only own considerable catalytic activity for CO2 reforming reaction but are also suitable for large-scale applications. The performance of 5%La-10%Co/Al2O3 catalyst in CO2 reforming reaction at various reaction temperatures while alternating initial partial pressure of CH4 and CO2 have been studied. The catalytic evaluation revealed that the excessive presence of CH4 could facilitate the direct CH4 decomposition resulting in catalyst active site blockage. The increment of 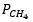 also gave rise to greater CH4 adsorption on the catalyst surface, consequently elevating the CO2 intake through the reaction. The increase of
also gave rise to greater CH4 adsorption on the catalyst surface, consequently elevating the CO2 intake through the reaction. The increase of 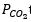 provoked the gasification rate improvement of deposited carbon from methane dissociation, therefore motivating the CH4 conversion. The consumption rate of CH4 was evidenced to be more sensitive toward the changes in reaction temperature than CO2. The CO2 reforming reaction performed over 5%La-10%Co/Al2O3 catalyst was convinced to follow an associative adsorption mode of CH4 and CO2 on dual or different active sites and the catalyst exhibited a good stability during 48 h reaction at 1023 K.
provoked the gasification rate improvement of deposited carbon from methane dissociation, therefore motivating the CH4 conversion. The consumption rate of CH4 was evidenced to be more sensitive toward the changes in reaction temperature than CO2. The CO2 reforming reaction performed over 5%La-10%Co/Al2O3 catalyst was convinced to follow an associative adsorption mode of CH4 and CO2 on dual or different active sites and the catalyst exhibited a good stability during 48 h reaction at 1023 K.

